CALORIMETRY_Part 01
For more information:
http://www.7activestudio.com
info@7activestudio.com
http://www.7activemedical.com/
info@7activemedical.com
http://www.sciencetuts.com/
7activestudio@gmail.com
Contact: +91- 9700061777,
040-64501777 / 65864777
7 Active Technology Solutions Pvt.Ltd. is an educational 3D digital content provider for K-12. We also customise the content as per your requirement for companies platform providers colleges etc . 7 Active driving force "The Joy of Happy Learning" -- is what makes difference from other digital content providers. We consider Student needs, Lecturer needs and College needs in designing the 3D & 2D Animated Video Lectures. We are carrying a huge 3D Digital Library ready to use.
CALORIMETRY: Calorimetry is one of the method for the determination of specific heat or latent heat of substance, the method used is method of mixtures.According to the principle of calorimetery,When a body at higher temperature is brought in contact with another body at lower temperature, the heat lost by the hot body is equal to the heat gain by the colder body, provided no heat is allowed to escape to the surroundings. Heat lost by the hot body = Heat gain by the cold body.This is known as principle of method of mixtures.CALORIMETER:-The device in which heat measurement can be made by utilising the principle of calorimetry is called a calorimeter.1.Calorimeter consists of cylindrical metallic vessel and stirrer of same material like copper or aluminium.2.The vessel is kept inside a wooden box, its outer surfaces are polished to prevent loss of heat due to radiation.3.The space between the calorimeter and wooden box is filled with non-conducting material like glass or wool to prevent heat loss due to conduction.4.Calorimeter is closed with a non-conducting lid to minimize the loss of heat due to convection.5. The stirrer and thermometer are inserted through a hole provided at the centre of a lid.EXPERIMENT: DETERMINATION OF SPECIFIC HEAT OF SOLID:PROCEDURE:-1.An empty and dry calorimeter with stirrer is cleaned and its mass is determined by using common balance.2.Water is filled in the calorimeter up to one-third of its volume and its mass is again determined.3.The calometer is placed in wooden box and temperature of water is noted as t1 degree centigrade using thermometer.4.Now the solid material whose specific heat is to be measured, taken in the form of small pieces is kept is a steam beater and then it is heated.5.The solid pieces are heated to a temperature t2 degree centigrade in steam heater.6.Now the solid pieces were quickly transferred into the calorimeter.7.The contents in the calorimeter are stirrer well and the resultant temperature of mixture T3 degree centigrade is noted.8.Finally, the mass of calorimeter along with water and solid pieces is determined.
THEORY:- Mass of calorimeter with stirrer = m1
Mass of calorimeter + stirrer + water = m2, Mass of water = m2 -m1
Mass of calorimeter + stirrer + water + solid = m3
Mass of solid = m3 -m2, initial temperature of calorimeter with water = t1 0c
Temperature of solid = t2 0c, Resultant temperature of mixture = t3 0c
Specific heat capacity of material of calorimeter = s1
Specific heat of water = s2, specific heat of solid = s
Amount heat gain by calorimeter = m1 s1 into t3 - t1
Amount of heat gain by water = m2 - m1 into s2 into t3 -- t1
Amount of heat gain by solid = m3 -m2) s t2 - t3
According to principle of method of mixtures
Heat lost by hot body = Heat gain by calorimeter and water
m3 - m2 s t2 - t 3 = m1 s1 +m2 --m1 into t3 - t1
Видео CALORIMETRY_Part 01 канала 7activestudio
http://www.7activestudio.com
info@7activestudio.com
http://www.7activemedical.com/
info@7activemedical.com
http://www.sciencetuts.com/
7activestudio@gmail.com
Contact: +91- 9700061777,
040-64501777 / 65864777
7 Active Technology Solutions Pvt.Ltd. is an educational 3D digital content provider for K-12. We also customise the content as per your requirement for companies platform providers colleges etc . 7 Active driving force "The Joy of Happy Learning" -- is what makes difference from other digital content providers. We consider Student needs, Lecturer needs and College needs in designing the 3D & 2D Animated Video Lectures. We are carrying a huge 3D Digital Library ready to use.
CALORIMETRY: Calorimetry is one of the method for the determination of specific heat or latent heat of substance, the method used is method of mixtures.According to the principle of calorimetery,When a body at higher temperature is brought in contact with another body at lower temperature, the heat lost by the hot body is equal to the heat gain by the colder body, provided no heat is allowed to escape to the surroundings. Heat lost by the hot body = Heat gain by the cold body.This is known as principle of method of mixtures.CALORIMETER:-The device in which heat measurement can be made by utilising the principle of calorimetry is called a calorimeter.1.Calorimeter consists of cylindrical metallic vessel and stirrer of same material like copper or aluminium.2.The vessel is kept inside a wooden box, its outer surfaces are polished to prevent loss of heat due to radiation.3.The space between the calorimeter and wooden box is filled with non-conducting material like glass or wool to prevent heat loss due to conduction.4.Calorimeter is closed with a non-conducting lid to minimize the loss of heat due to convection.5. The stirrer and thermometer are inserted through a hole provided at the centre of a lid.EXPERIMENT: DETERMINATION OF SPECIFIC HEAT OF SOLID:PROCEDURE:-1.An empty and dry calorimeter with stirrer is cleaned and its mass is determined by using common balance.2.Water is filled in the calorimeter up to one-third of its volume and its mass is again determined.3.The calometer is placed in wooden box and temperature of water is noted as t1 degree centigrade using thermometer.4.Now the solid material whose specific heat is to be measured, taken in the form of small pieces is kept is a steam beater and then it is heated.5.The solid pieces are heated to a temperature t2 degree centigrade in steam heater.6.Now the solid pieces were quickly transferred into the calorimeter.7.The contents in the calorimeter are stirrer well and the resultant temperature of mixture T3 degree centigrade is noted.8.Finally, the mass of calorimeter along with water and solid pieces is determined.
THEORY:- Mass of calorimeter with stirrer = m1
Mass of calorimeter + stirrer + water = m2, Mass of water = m2 -m1
Mass of calorimeter + stirrer + water + solid = m3
Mass of solid = m3 -m2, initial temperature of calorimeter with water = t1 0c
Temperature of solid = t2 0c, Resultant temperature of mixture = t3 0c
Specific heat capacity of material of calorimeter = s1
Specific heat of water = s2, specific heat of solid = s
Amount heat gain by calorimeter = m1 s1 into t3 - t1
Amount of heat gain by water = m2 - m1 into s2 into t3 -- t1
Amount of heat gain by solid = m3 -m2) s t2 - t3
According to principle of method of mixtures
Heat lost by hot body = Heat gain by calorimeter and water
m3 - m2 s t2 - t 3 = m1 s1 +m2 --m1 into t3 - t1
Видео CALORIMETRY_Part 01 канала 7activestudio
Показать
Комментарии отсутствуют
Информация о видео
Другие видео канала
 CALORIMETRY _ Part 02
CALORIMETRY _ Part 02 Calorimetry
Calorimetry Bomb Calorimeter
Bomb Calorimeter Specific Heat of a Metal Lab
Specific Heat of a Metal Lab Calorimeter
Calorimeter Calorimeter | 10th Std | Physics | ICSE Board | Home Revise
Calorimeter | 10th Std | Physics | ICSE Board | Home Revise Introduction to Colorimeter || Demonstration of Colorimeter Practical || Beer Lambert's Law
Introduction to Colorimeter || Demonstration of Colorimeter Practical || Beer Lambert's Law How To Produce Oxygen Gas At Home/OXYGEN AND HYDROGEN From Water | Electrolysis Of Water At Home
How To Produce Oxygen Gas At Home/OXYGEN AND HYDROGEN From Water | Electrolysis Of Water At Home
 Heat Capacity, Specific Heat, and Calorimetry
Heat Capacity, Specific Heat, and Calorimetry Coffee Cup Calorimetry
Coffee Cup Calorimetry Specific Heat Capacity of Water Experiment - GCSE Physics Required Practical
Specific Heat Capacity of Water Experiment - GCSE Physics Required Practical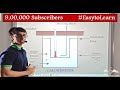 Calorimetry
Calorimetry Thermal Properties Of Matter 03 || Calorimetry - Compilation of Old Videos ||
Thermal Properties Of Matter 03 || Calorimetry - Compilation of Old Videos || Calorimetry - Determination of Heats of Solution
Calorimetry - Determination of Heats of Solution Coffee Cup Calorimetry
Coffee Cup Calorimetry Determination of Viscosity Using Ostwald Viscometer (With observation and Calculation) HINDI
Determination of Viscosity Using Ostwald Viscometer (With observation and Calculation) HINDI Calorimeter | Reactions | Chemistry | FuseSchool
Calorimeter | Reactions | Chemistry | FuseSchool Class 11 chemistry Thermodynamics (calorific value of fuel and Bomb calorimeter)
Class 11 chemistry Thermodynamics (calorific value of fuel and Bomb calorimeter) Specific Heat Capacity | Matter | Physics | FuseSchool
Specific Heat Capacity | Matter | Physics | FuseSchool