Half Reaction Method, Balancing Redox Reactions In Basic & Acidic Solution, Chemistry
This chemistry video tutorial provides a basic introduction into the half reaction method which is useful for balancing redox reactions in basic solution and in acidic solution. This video shows all the steps needed to balance the oxidation reduction reaction. First, separate the reaction into two half reactions. Balance the number of atoms and the charge on both sides. In acidic solution, you can add H+ and H2O to balance the particles. In Basic solution, you can use OH- and H2O to do so. Add electrons to the side with the highest charge to balance the total charge on both sides of the chemical equation. Make sure the number of electrons are the same on both half reactions before adding the two to get the net reaction and that's it. This video contains plenty of examples and practice problems.
My E-Book: https://amzn.to/3B9c08z
Video Playlists: https://www.video-tutor.net
Homework Help: https://bit.ly/Find-A-Tutor
Subscribe: https://bit.ly/37WGgXl
Support & Donations: https://www.patreon.com/MathScienceTutor
Youtube Membership: https://www.youtube.com/channel/UCEWpbFLzoYGPfuWUMFPSaoA/join
New Chemistry Video Playlist:
https://www.youtube.com/watch?v=bka20Q9TN6M&t=25s&list=PL0o_zxa4K1BWziAvOKdqsMFSB_MyyLAqS&index=1
Видео Half Reaction Method, Balancing Redox Reactions In Basic & Acidic Solution, Chemistry канала The Organic Chemistry Tutor
My E-Book: https://amzn.to/3B9c08z
Video Playlists: https://www.video-tutor.net
Homework Help: https://bit.ly/Find-A-Tutor
Subscribe: https://bit.ly/37WGgXl
Support & Donations: https://www.patreon.com/MathScienceTutor
Youtube Membership: https://www.youtube.com/channel/UCEWpbFLzoYGPfuWUMFPSaoA/join
New Chemistry Video Playlist:
https://www.youtube.com/watch?v=bka20Q9TN6M&t=25s&list=PL0o_zxa4K1BWziAvOKdqsMFSB_MyyLAqS&index=1
Видео Half Reaction Method, Balancing Redox Reactions In Basic & Acidic Solution, Chemistry канала The Organic Chemistry Tutor
Показать
Комментарии отсутствуют
Информация о видео
23 августа 2017 г. 16:00:03
00:16:00
Другие видео канала
 Balancing Redox Reactions in Acidic and Basic Conditions
Balancing Redox Reactions in Acidic and Basic Conditions Oxidation-Reduction Reactions
Oxidation-Reduction Reactions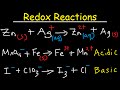 How To Balance Redox Reactions - General Chemistry Practice Test / Exam Review
How To Balance Redox Reactions - General Chemistry Practice Test / Exam Review Introduction to Galvanic Cells & Voltaic Cells
Introduction to Galvanic Cells & Voltaic Cells How to Balance Redox Equations in Acidic Solution
How to Balance Redox Equations in Acidic Solution How To Calculate Oxidation Numbers - Basic Introduction
How To Calculate Oxidation Numbers - Basic Introduction
 Oxidizing Agents and Reducing Agents
Oxidizing Agents and Reducing Agents How To Write Net Ionic Equations In Chemistry - A Simple Method!
How To Write Net Ionic Equations In Chemistry - A Simple Method! Oxidation and Reduction Reactions - Basic Introduction
Oxidation and Reduction Reactions - Basic Introduction Redox Reactions: Crash Course Chemistry #10
Redox Reactions: Crash Course Chemistry #10 Math Has a Fatal Flaw
Math Has a Fatal Flaw Cell Potential Problems - Electrochemistry
Cell Potential Problems - Electrochemistry Half Equations Tutorial
Half Equations Tutorial Precipitation Reactions & Net Ionic Equations - Chemistry
Precipitation Reactions & Net Ionic Equations - Chemistry Nernst Equation Explained, Electrochemistry, Example Problems, pH, Chemistry, Galvanic Cell
Nernst Equation Explained, Electrochemistry, Example Problems, pH, Chemistry, Galvanic Cell Balancing Redox Reactions with Half Reaction Method
Balancing Redox Reactions with Half Reaction Method How to Find Oxidation Numbers (Rules and Examples)
How to Find Oxidation Numbers (Rules and Examples) Chemistry 13.4 Writing Half-reactions for Redox
Chemistry 13.4 Writing Half-reactions for Redox Electrochemistry Review - Cell Potential & Notation, Redox Half Reactions, Nernst Equation
Electrochemistry Review - Cell Potential & Notation, Redox Half Reactions, Nernst Equation