Balancing Redox Reactions in Acidic and Basic Conditions
We know that redox reactions are ones that involve electron transfer. Something is oxidized, and something else is reduced. But these reactions can be tricky to balance when surrounding water molecules are involved in the reaction. Let's go through the algorithm for balancing redox reactions in both acidic and basic conditions.
Watch the whole General Chemistry playlist: http://bit.ly/ProfDaveGenChem
Organic Chemistry Tutorials: http://bit.ly/ProfDaveOrgChem
Biochemistry Tutorials: http://bit.ly/ProfDaveBiochem
Biology Tutorials: http://bit.ly/ProfDaveBio
Classical Physics Tutorials: http://bit.ly/ProfDavePhysics1
Modern Physics Tutorials: http://bit.ly/ProfDavePhysics2
Mathematics Tutorials: http://bit.ly/ProfDaveMaths
EMAIL► ProfessorDaveExplains@gmail.com
PATREON► http://patreon.com/ProfessorDaveExplains
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Amazon: https://amzn.to/2HtNpVH
Bookshop: https://bit.ly/39cKADM
Barnes and Noble: https://bit.ly/3pUjmrn
Book Depository: http://bit.ly/3aOVDlT
Видео Balancing Redox Reactions in Acidic and Basic Conditions канала Professor Dave Explains
Watch the whole General Chemistry playlist: http://bit.ly/ProfDaveGenChem
Organic Chemistry Tutorials: http://bit.ly/ProfDaveOrgChem
Biochemistry Tutorials: http://bit.ly/ProfDaveBiochem
Biology Tutorials: http://bit.ly/ProfDaveBio
Classical Physics Tutorials: http://bit.ly/ProfDavePhysics1
Modern Physics Tutorials: http://bit.ly/ProfDavePhysics2
Mathematics Tutorials: http://bit.ly/ProfDaveMaths
EMAIL► ProfessorDaveExplains@gmail.com
PATREON► http://patreon.com/ProfessorDaveExplains
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Amazon: https://amzn.to/2HtNpVH
Bookshop: https://bit.ly/39cKADM
Barnes and Noble: https://bit.ly/3pUjmrn
Book Depository: http://bit.ly/3aOVDlT
Видео Balancing Redox Reactions in Acidic and Basic Conditions канала Professor Dave Explains
Показать
Комментарии отсутствуют
Информация о видео
13 декабря 2018 г. 21:49:47
00:07:31
Другие видео канала
 How to Balance Redox Equations in Acidic Solution
How to Balance Redox Equations in Acidic Solution Electrochemistry
Electrochemistry Oxidation-Reduction Reactions
Oxidation-Reduction Reactions How To Balance Redox Equations In Basic Solution
How To Balance Redox Equations In Basic Solution Redox Reactions: Crash Course Chemistry #10
Redox Reactions: Crash Course Chemistry #10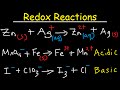 How To Balance Redox Reactions - General Chemistry Practice Test / Exam Review
How To Balance Redox Reactions - General Chemistry Practice Test / Exam Review
 Trick for Balancing Redox Reactions in Acidic Medium
Trick for Balancing Redox Reactions in Acidic Medium My Landlord is a Scammer!
My Landlord is a Scammer! Balancing Redox Reactions-Acidic Conditions
Balancing Redox Reactions-Acidic Conditions Introduction to Oxidation Reduction (Redox) Reactions
Introduction to Oxidation Reduction (Redox) Reactions Half Reaction Method, Balancing Redox Reactions In Basic & Acidic Solution, Chemistry
Half Reaction Method, Balancing Redox Reactions In Basic & Acidic Solution, Chemistry Half Reaction Method
Half Reaction Method Balancing Redox Reactions, Galvanic Cells, Finding Cell Potential, & Cell Notation
Balancing Redox Reactions, Galvanic Cells, Finding Cell Potential, & Cell Notation How to Balance Redox Equations in Basic Solution
How to Balance Redox Equations in Basic Solution Quantum Numbers, Atomic Orbitals, and Electron Configurations
Quantum Numbers, Atomic Orbitals, and Electron Configurations Oxidizing Agents and Reducing Agents
Oxidizing Agents and Reducing Agents Trick to Balance disproportionation Reaction with 5 simple steps both in Acidic & Basic medium
Trick to Balance disproportionation Reaction with 5 simple steps both in Acidic & Basic medium How to Find Oxidation Numbers (Rules and Examples)
How to Find Oxidation Numbers (Rules and Examples) Redox Reactions
Redox Reactions