Surface Tension of Water, Capillary Action, Cohesive and Adhesive Forces - Work & Potential Energy
This physics video tutorial provides a basic introduction into the surface tension of water. Surface tension prevents small amounts of water from flattening out across a surface. Rather, it causes water to minimize its surface area and as a result, water forms small beadlike droplets. This video also discusses the capillary action of water as it is draw up through small tubes known as capillaries. Surface tension can be calculated by divided the force per unit length required to increase the area of a fluid. The surface tension opposes the force and attempts to minimize the area. The work required to increase the area of a fluid by a force is the surface tension multiplied by the increase in area. This video discusses the difference in the capillary effects between water and mercury. Water rises above the surrounding fluid level in a thin glass tube where as mercury descends below that line. The adhesive forces in water is greater than the cohesive forces. In Mercury, the cohesive forces are greater than the adhesive forces. Adhesive forces exist between different molecules and atoms. Cohesive forces exist between the same type of molecules and atoms.
New Physics Video Playlist:
https://www.youtube.com/playlist?list=PL0o_zxa4K1BU6wPPLDsoTj1_wEf0LSNeR
Access to Premium Videos:
https://www.patreon.com/MathScienceTutor
https://www.facebook.com/MathScienceTutoring/
Видео Surface Tension of Water, Capillary Action, Cohesive and Adhesive Forces - Work & Potential Energy канала The Organic Chemistry Tutor
New Physics Video Playlist:
https://www.youtube.com/playlist?list=PL0o_zxa4K1BU6wPPLDsoTj1_wEf0LSNeR
Access to Premium Videos:
https://www.patreon.com/MathScienceTutor
https://www.facebook.com/MathScienceTutoring/
Видео Surface Tension of Water, Capillary Action, Cohesive and Adhesive Forces - Work & Potential Energy канала The Organic Chemistry Tutor
Показать
Комментарии отсутствуют
Информация о видео
17 ноября 2017 г. 17:00:01
00:12:54
Другие видео канала
 Surface Tension and Adhesion | Fluids | Physics | Khan Academy
Surface Tension and Adhesion | Fluids | Physics | Khan Academy Viscosity of Fluids & Velocity Gradient - Fluid Mechanics, Physics Problems
Viscosity of Fluids & Velocity Gradient - Fluid Mechanics, Physics Problems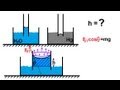 Physics 33 - Fluid Statics - Surface Tension (5 of 12): What Causes Capillary Action?
Physics 33 - Fluid Statics - Surface Tension (5 of 12): What Causes Capillary Action? Capillary action and why we see a meniscus | Chemistry | Khan Academy
Capillary action and why we see a meniscus | Chemistry | Khan Academy Phase Diagrams of Water & CO2 Explained - Chemistry - Melting, Boiling & Critical Point
Phase Diagrams of Water & CO2 Explained - Chemistry - Melting, Boiling & Critical Point
 What is Surface Tension? | Richard Hammond's Invisible Worlds | Earth Lab
What is Surface Tension? | Richard Hammond's Invisible Worlds | Earth Lab Viscosity, Cohesive and Adhesive Forces, Surface Tension, and Capillary Action
Viscosity, Cohesive and Adhesive Forces, Surface Tension, and Capillary Action Archimedes Principle, Buoyant Force, Basic Introduction - Buoyancy & Density - Fluid Statics
Archimedes Principle, Buoyant Force, Basic Introduction - Buoyancy & Density - Fluid Statics How ELECTRICITY works - working principle
How ELECTRICITY works - working principle Science Mom's Guide to Water, Part 3 - Capillary Action
Science Mom's Guide to Water, Part 3 - Capillary Action Surface Tension
Surface Tension Surface tension
Surface tension Fluid Pressure, Density, Archimede & Pascal's Principle, Buoyant Force, Bernoulli's Equation Physics
Fluid Pressure, Density, Archimede & Pascal's Principle, Buoyant Force, Bernoulli's Equation Physics What math and science cannot (yet?) explain
What math and science cannot (yet?) explain Cohesive and Adhesive Forces of Water
Cohesive and Adhesive Forces of Water Thermodynamics, PV Diagrams, Internal Energy, Heat, Work, Isothermal, Adiabatic, Isobaric, Physics
Thermodynamics, PV Diagrams, Internal Energy, Heat, Work, Isothermal, Adiabatic, Isobaric, Physics Understanding Bernoulli's Equation
Understanding Bernoulli's Equation Latent Heat of Fusion and Vaporization, Specific Heat Capacity & Calorimetry - Physics
Latent Heat of Fusion and Vaporization, Specific Heat Capacity & Calorimetry - Physics IRR (Internal Rate of Return)
IRR (Internal Rate of Return)