Boiling Point of an Organic Compound - MeitY OLabs
This video channel is developed by Amrita University's CREATE
http://www.amrita.edu/create
▶For more Information @
http://amrita.olabs.co.in/?sub=73&brch=7&sim=111&cnt=1
▶ Online Labs for School lab Experiments (Olabs)
http://www.olabs.edu.in/
▶ Learn more about Amrita University
http://www.amrita.edu
▶ Subscribe @
http://www.youtube.com/amritacreate
https://www.facebook.com/onlinelabs
Copyright © 2013 Amrita University
Developed by CDAC Mumbai & Amrita University under research grant from Department of IT, Government of India
Boiling Point of an Organic Compound :-
The boiling point of organic compounds can give important information about their physical properties and structural characteristics. Boiling point helps identify and characterise a compound. A liquid boils when its vapour pressure is equal to the atmospheric pressure. Vapour pressure is determined by the kinetic energy of a molecule. The boiling point of a liquid varies with the surrounding atmospheric pressure. A liquid at a higher pressure has a higher boiling point than when that liquid is at lower atmospheric pressure. The boiling point (BP) of an organic molecule is related to the molecular weight of the molecule and the "stickiness" of individual molecules for their neighbors. For molecules with a given functional group, boiling point increases with molecular weight.
This video explains how to determine the boiling point of an organic compound.
Видео Boiling Point of an Organic Compound - MeitY OLabs канала amritacreate
http://www.amrita.edu/create
▶For more Information @
http://amrita.olabs.co.in/?sub=73&brch=7&sim=111&cnt=1
▶ Online Labs for School lab Experiments (Olabs)
http://www.olabs.edu.in/
▶ Learn more about Amrita University
http://www.amrita.edu
▶ Subscribe @
http://www.youtube.com/amritacreate
https://www.facebook.com/onlinelabs
Copyright © 2013 Amrita University
Developed by CDAC Mumbai & Amrita University under research grant from Department of IT, Government of India
Boiling Point of an Organic Compound :-
The boiling point of organic compounds can give important information about their physical properties and structural characteristics. Boiling point helps identify and characterise a compound. A liquid boils when its vapour pressure is equal to the atmospheric pressure. Vapour pressure is determined by the kinetic energy of a molecule. The boiling point of a liquid varies with the surrounding atmospheric pressure. A liquid at a higher pressure has a higher boiling point than when that liquid is at lower atmospheric pressure. The boiling point (BP) of an organic molecule is related to the molecular weight of the molecule and the "stickiness" of individual molecules for their neighbors. For molecules with a given functional group, boiling point increases with molecular weight.
This video explains how to determine the boiling point of an organic compound.
Видео Boiling Point of an Organic Compound - MeitY OLabs канала amritacreate
Показать
Комментарии отсутствуют
Информация о видео
Другие видео канала
 Melting Point of an Organic Compound - MeitY OLabs
Melting Point of an Organic Compound - MeitY OLabs
 Purification of Copper Sulphate by Crystallization - MeitY OLabs
Purification of Copper Sulphate by Crystallization - MeitY OLabs Boiling Point of an Organic compound - MeitY OLabs
Boiling Point of an Organic compound - MeitY OLabs Boiling Point of Organic Compounds
Boiling Point of Organic Compounds Making Soap
Making Soap Determination of boiling point
Determination of boiling point Determination of Melting Point
Determination of Melting Point How To Measure A Micro Boiling Point
How To Measure A Micro Boiling Point Lab 1. Part 2. Boiling Point
Lab 1. Part 2. Boiling Point BOILING POINT OF LIQUIDS
BOILING POINT OF LIQUIDS A Simple Distillation Explained
A Simple Distillation Explained Detection of Elements: Lassaigne’s Test - MeitY OLabs
Detection of Elements: Lassaigne’s Test - MeitY OLabs Melting Point of an Organic Compound - MeitY OLabs
Melting Point of an Organic Compound - MeitY OLabs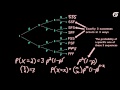 An Introduction to the Binomial Distribution
An Introduction to the Binomial Distribution Cheek Epithelial Cells: How to Prepare a Wet Mount Microscope Slide
Cheek Epithelial Cells: How to Prepare a Wet Mount Microscope Slide Quantitative Estimation - MeitY OLabs
Quantitative Estimation - MeitY OLabs In A World of Systems
In A World of Systems Boiling Point of Water | Learn with BYJU'S
Boiling Point of Water | Learn with BYJU'S How to Determine Melting Point/Boiling point of Organic Compound
How to Determine Melting Point/Boiling point of Organic Compound