Calculating the energy for the singlet and triplet states of a carbon atom
All calculations were carried out using the B3LYP/6-311++G(d,p) method. The Gaussian software Windows version is used. Hund's rule is verified. Courtesy of Melissa Countryman.
Hund's rule states that when multiple electrons occupy degenerate atomic orbitals, the atomic energy is minimized when the total spin of these electrons are maximized. This is because two electrons with the same spin cannot appear in the same position (quantum effect) and thus have smaller repulsion between each other than two electrons with opposite spins.
Видео Calculating the energy for the singlet and triplet states of a carbon atom канала Physical Chemistry (PChem)
Hund's rule states that when multiple electrons occupy degenerate atomic orbitals, the atomic energy is minimized when the total spin of these electrons are maximized. This is because two electrons with the same spin cannot appear in the same position (quantum effect) and thus have smaller repulsion between each other than two electrons with opposite spins.
Видео Calculating the energy for the singlet and triplet states of a carbon atom канала Physical Chemistry (PChem)
Показать
Комментарии отсутствуют
Информация о видео
8 февраля 2014 г. 1:09:00
00:05:13
Другие видео канала
 35 4 Reaction Mechanisms
35 4 Reaction Mechanisms 6 13 Le Chatelier Principle
6 13 Le Chatelier Principle Integrated rate law for second-order A+B=P reversible reactions
Integrated rate law for second-order A+B=P reversible reactions 1 5 vdW Gas
1 5 vdW Gas 22 14 Cyclopentadiene Cycloheptatriene
22 14 Cyclopentadiene Cycloheptatriene 7 8 Tetrahedral Complexes Chirality
7 8 Tetrahedral Complexes Chirality Chem182 Summary of First Day
Chem182 Summary of First Day RRK Theory - Energy Distribution
RRK Theory - Energy Distribution 2021-8-26 Ocean Shores, Washington
2021-8-26 Ocean Shores, Washington 18 6 Quantization of Angular Momentum
18 6 Quantization of Angular Momentum Replacing carpet with laminated wood 5. Trim the door frame.
Replacing carpet with laminated wood 5. Trim the door frame. 21 7 Base Hydrolysis
21 7 Base Hydrolysis Deriving the Maxwells Relations
Deriving the Maxwells Relations Gas 5 Mean Free Path Diffusion Effusion
Gas 5 Mean Free Path Diffusion Effusion 2 2 Work
2 2 Work Determining Eutectic Point of NaK Alloy
Determining Eutectic Point of NaK Alloy 35 14 Activated Complex Theory
35 14 Activated Complex Theory Titrating Acetic Acid Using Vernier pH Sensor
Titrating Acetic Acid Using Vernier pH Sensor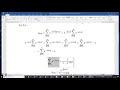 Deriving the Vibrational Energy Given the Partition Function
Deriving the Vibrational Energy Given the Partition Function EXP #1 Methyl Red Lecture
EXP #1 Methyl Red Lecture 26 Computational Chemistry
26 Computational Chemistry