Bonding and Lewis Structures (English)
Chapter 1: Why and How a Bond will Form, Predicting Formation of an Ionic, Covalent, and Polar Covalent Bond from Differences of Electronegativity Values, Electronegativity Values and Periodic Trends, Relating Full Valency to Abbreviated Electron Configurations, Internuclear Distance vs. Energy Diagrams, Bond Distances, Dipoles, Shielding and De-shielding
Chapter 2: Lewis Dot Diagrams, Rules for Drawing Lewis Structures, Octet and Duet Rule, Single, Double, and Triple Bond, Many Examples Worked for the Student.
Chapter 3: General Rules and Exceptions to the Octet Rule, Many Examples for Exceptions to the Octet Rule.
Chapter 4: Organic Compounds, Alternate Approach to Drawing Lewis Structures, Calculating Number of Bonds Needed for an Atom, Constitutional or Structural Isomers, Geometric Isomers (E and Z), Cahn-Ingold Prelog Sequence Rules, Degrees of Unsaturation to Develop a Logical Step-Wise Approach to Drawing all Isomers, Alkanes, Alkenes, Alkynes, Cyclic and Acyclic Structures, Deducing Equivalent Hydrogens, Oxygen and Sulfur Insertion, Odd Nitrogen Rule, Over 40 Examples Worked for the Student.
Narration: Gordon W. Gribble, PH.D., Dartmouth College
The DVD version is available at: https://sponholtzproductions.com/
Видео Bonding and Lewis Structures (English) канала PassChem: Sponholtz Productions
Chapter 2: Lewis Dot Diagrams, Rules for Drawing Lewis Structures, Octet and Duet Rule, Single, Double, and Triple Bond, Many Examples Worked for the Student.
Chapter 3: General Rules and Exceptions to the Octet Rule, Many Examples for Exceptions to the Octet Rule.
Chapter 4: Organic Compounds, Alternate Approach to Drawing Lewis Structures, Calculating Number of Bonds Needed for an Atom, Constitutional or Structural Isomers, Geometric Isomers (E and Z), Cahn-Ingold Prelog Sequence Rules, Degrees of Unsaturation to Develop a Logical Step-Wise Approach to Drawing all Isomers, Alkanes, Alkenes, Alkynes, Cyclic and Acyclic Structures, Deducing Equivalent Hydrogens, Oxygen and Sulfur Insertion, Odd Nitrogen Rule, Over 40 Examples Worked for the Student.
Narration: Gordon W. Gribble, PH.D., Dartmouth College
The DVD version is available at: https://sponholtzproductions.com/
Видео Bonding and Lewis Structures (English) канала PassChem: Sponholtz Productions
Показать
Комментарии отсутствуют
Информация о видео
13 марта 2019 г. 5:07:05
00:42:05
Другие видео канала

 Hybridization Theory (English)
Hybridization Theory (English) Lewis Structures, Introduction, Formal Charge, Molecular Geometry, Resonance, Polar or Nonpolar
Lewis Structures, Introduction, Formal Charge, Molecular Geometry, Resonance, Polar or Nonpolar 10. Lewis Structures
10. Lewis Structures This Is How We Do It.. CO2 hybridization with Elizabeth
This Is How We Do It.. CO2 hybridization with Elizabeth Introduction to the Atom (English)
Introduction to the Atom (English) Why do atoms form molecules? The quantum physics of chemical bonds explained
Why do atoms form molecules? The quantum physics of chemical bonds explained Bonding Models and Lewis Structures: Crash Course Chemistry #24
Bonding Models and Lewis Structures: Crash Course Chemistry #24 Sharing Geology | Nick Zentner | TEDxYakimaSalon
Sharing Geology | Nick Zentner | TEDxYakimaSalon Investigating the Periodic Table with Experiments - with Peter Wothers
Investigating the Periodic Table with Experiments - with Peter Wothers Types of Orbitals: d-orbitals
Types of Orbitals: d-orbitals Understanding the Atom: Intro Quantum and Electron Configurations (English)
Understanding the Atom: Intro Quantum and Electron Configurations (English) Goodbye Determinism, Hello Heisenberg Uncertainty Principle
Goodbye Determinism, Hello Heisenberg Uncertainty Principle Phenylethylamine (PEA)
Phenylethylamine (PEA) 4.6 Quantum Mechanics and Bonding: Hybridization
4.6 Quantum Mechanics and Bonding: Hybridization Atomic Structure: Protons, Electrons & Neutrons
Atomic Structure: Protons, Electrons & Neutrons Introduction to Ionic Bonding and Covalent Bonding
Introduction to Ionic Bonding and Covalent Bonding Atoms and Molecules (an animated lecture video)
Atoms and Molecules (an animated lecture video)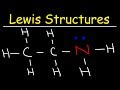 Organic Chemistry - How To Draw Lewis Structures
Organic Chemistry - How To Draw Lewis Structures Types of Nuclear Radiation
Types of Nuclear Radiation